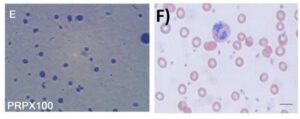
Thanks to innovative gel-barrier separation technology, Tropocells® PRP can reliably deliver the optimal PRP formula of 100% removal of RBCs, 93% removal of neutrophils, and preserving the monocytes and lymphocytes. This is an innovative and technological breakthrough that has made Tropocells® PRP the new golden standard for PRP systems.
Unique patented gel allows to separate essential growth and regeneration factors from harmful an unnecessary blood cells such as RBCs and neutrophils. This means no painful inflammatory flare to patients. It also means a potential for better clinical outcomes. Clinical experience shows that inflammatory flares may cause patients to limp around in pain for days. Patients who are treated with Tropocells® PRP system enjoy a relatively painless procedure and experience more positive and longer-lasting outcomes.
It should also be noted that Tropocells® has other distinct advantages over other PRP systems that indirectly improve patient outcomes:
- Buffered anticoagulant
- Higher platelet yield
- Less pain during injection
- Specially-treated collection tubes
- Higher platelet yield
- Less risk of premature platelet activation
- Simple preparation process within a closed system (PRP is generated in the same blood collection tube)
- Higher platelet yield
- Less risk of bacterial contamination
- Less risk of needle-stick injury